The information provided in this section is intended expressly for healthcare professionals in the United States. Click “OK” to enter if you are a US healthcare professional.
THE MAJORITY OF ADVERSE REACTIONS IN ≥10% OF ADULT AND PEDIATRIC PATIENTS WERE GRADE 1 OR 21
Low incidence of Grade 3 or 4 adverse reactions occurring in ≥10% of patients1
Adverse Reactions Occurring in ≥10% of Patients Treated With VITRAKVI®1
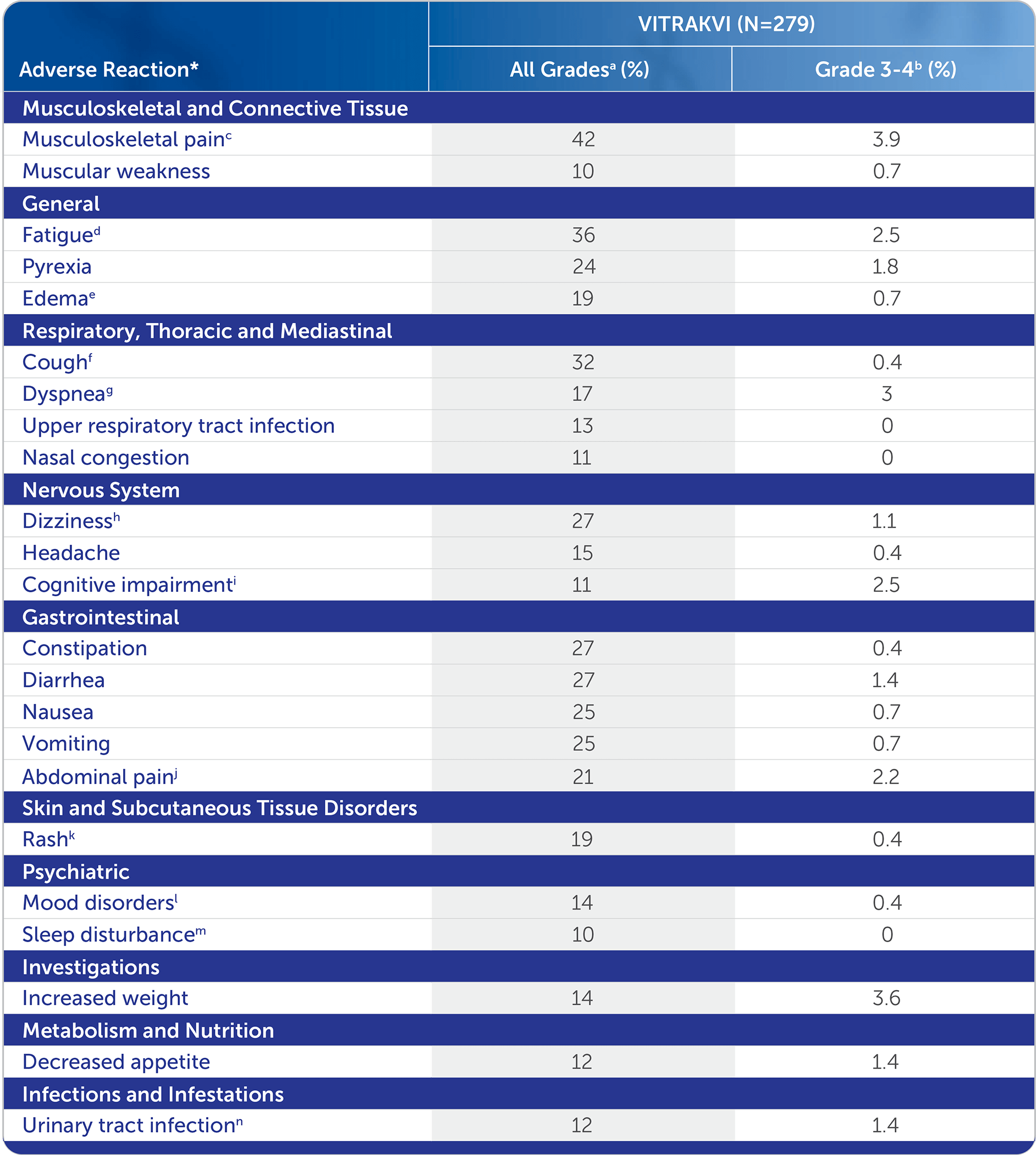
*The adverse reaction identifies a composite term.
aNational Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.03.
bOne Grade 4 adverse reaction of pyrexia.
cIncludes: arthralgia, back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, and pain in extremity.
dIncludes: fatigue, asthenia.
eIncludes: face edema, generalized edema, lip edema, localized edema, edema, edema genital, edema peripheral, periorbital edema, and swelling.
fIncludes: cough, productive cough, and upper-airway cough syndrome.
gIncludes: dyspnea, and dyspnea exertional.
hIncludes: dizziness, dizziness postural, and vertigo.
iIncludes: amnesia, aphasia, cognitive disorder, confusional state, delirium, disturbance in attention, hallucinations, memory impairment, mental impairment, mental status changes.
jIncludes: abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness, epigastric discomfort, and gastrointestinal pain.
kIncludes: dermatitis, dermatitis acneiform, dermatitis bullous, dermatitis exfoliative generalized, eczema, eczema asteatotic, palmar-plantar erythrodysesthesia syndrome, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular.
lIncludes: agitation, anxiety, depression, depressed mood, euphoric mood, irritability.
mIncludes: insomnia, sleep disorder, somnolence.
nIncludes: cystitis, escherichia urinary tract infection, pyelonephritis acute, and urinary tract infection.
Indication
VITRAKVI is indicated for the treatment of adult and pediatric patients with solid tumors that:
- have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation,
- are metastatic or where surgical resection is likely to result in severe morbidity, and
- have no satisfactory alternative treatments or that have progressed following treatment.
Select patients for therapy based on an FDA-approved test.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Important Safety Information
Warnings and Precautions
Central Nervous System Effects: Central nervous system (CNS) adverse reactions occurred in patients receiving VITRAKVI, including dizziness, cognitive impairment, mood disorders, and sleep disturbances.
In patients who received VITRAKVI, all grades CNS effects including cognitive impairment, mood disorders, dizziness and sleep disorders were observed in 42% with Grades 3-4 in 3.9% of patients.
Cognitive impairment occurred in 11% of patients. The median time to onset of cognitive impairment was 5.6 months (range: 2 days to 41 months). Cognitive impairment occurring in ≥1% of patients included memory impairment (3.6%), confusional state (2.9%), disturbance in attention (2.9%), delirium (2.2%), cognitive disorders (1.4%), and Grade 3 cognitive adverse reactions occurred in 2.5% of patients. Among the 30 patients with cognitive impairment, 7% required a dose modification and 20% required dose interruption.
Mood disorders occurred in 14% of patients. The median time to onset of mood disorders was 3.9 months (range: 1 day to 40.5 months). Mood disorders occurring in ≥1% of patients included anxiety (5%), depression (3.9%), agitation (2.9%), and irritability (2.9%). Grade 3 mood disorders occurred in 0.4% of patients.
Dizziness occurred in 27% of patients, and Grade 3 dizziness occurred in 1.1% of patients. Among the 74 patients who experienced dizziness, 5% of patients required a dose modification and 5% required dose interruption.
Sleep disturbances occurred in 10% of patients. Sleep disturbances included insomnia (7%), somnolence (2.5%), and sleep disorder (0.4%). There were no Grade 3-4 sleep disturbances. Among the 28 patients who experienced sleep disturbances, 1 patient each (3.6%) required a dose modification or dose interruption.
Advise patients and caretakers of these risks with VITRAKVI. Advise patients not to drive or operate hazardous machinery if they are experiencing neurologic adverse reactions. Withhold or permanently discontinue VITRAKVI based on the severity. If withheld, modify the VITRAKVI dosage when resumed.
Skeletal Fractures: Among 187 adult patients who received VITRAKVI across clinical trials, fractures were reported in 7% and among 92 pediatric patients, fractures were reported in 9% (N=279; 8%). Median time to fracture was 11.6 months (range 0.9 to 45.8 months) in patients followed per fracture. Fractures of the femur, hip or acetabulum were reported in 4 patients (3 adult, 1 pediatric). Most fractures were associated with minimal or moderate trauma. Some fractures were associated with radiologic abnormalities suggestive of local tumor involvement. VITRAKVI treatment was interrupted due to fracture in 1.4% patients.
Promptly evaluate patients with signs or symptoms of potential fracture (e.g., pain, changes in mobility, deformity). There are no data on the effects of VITRAKVI on healing of known fractures or risk of future fractures.
Hepatotoxicity: Hepatotoxicity including drug induced liver injury (DILI) has been reported in patients taking VITRAKVI.
In patients who received VITRAKVI, increased AST of any grade occurred in 52% of patients and increased ALT of any grade occurred in 45%. Grade 3-4 increased AST or ALT occurred in 3.1% and 2.5% of patients, respectively. The median time to onset of increased AST was 2.1 months (range: 1 day to 4.3 years). The median time to onset of increased ALT was 2.3 months (range: 1 day to 4.2 years). Increased AST and ALT leading to dose modifications occurred in 1.4% and 2.2% of patients, respectively. Increased AST or ALT led to permanent discontinuation in 3 (1.1%) of patients.
There have been reports in adult patients from clinical studies and post-marketing cases of Grade ≥ 2 increases in ALT and/or AST with increases in bilirubin ≥ 2 x ULN.
Obtain liver function tests (ALT, AST, ALP and bilirubin) before initiation of VITRAKVI and monitor every 2 weeks during the first two months of treatment, then monthly thereafter, or more frequently following the occurrence of Grade 2 or greater AST or ALT elevation. Temporarily withhold, reduce the dose, or permanently discontinue VITRAKVI based on severity.
Embryo-Fetal Toxicity: VITRAKVI can cause fetal harm when administered to a pregnant woman. Larotrectinib resulted in malformations in rats and rabbits at maternal exposures that were approximately 11- and 0.7-times, respectively, those observed at the clinical dose of 100 mg twice daily. Advise women of the potential risk to a fetus. Advise females of reproductive potential to use an effective method of contraception during treatment and for 1 week after the last dose of VITRAKVI.
Adverse Reactions
The most common adverse reactions (≥20%), including laboratory abnormalities, were: increased AST (52%), increased ALT (45%), anemia (42%), musculoskeletal pain (42%), fatigue (36%), hypoalbuminemia (36%), neutropenia (36%), increased alkaline phosphatase (34%), cough (32%), leukopenia (28%), constipation (27%), diarrhea (27%), dizziness (27%), hypocalcemia (25%), nausea (25%), vomiting (25%), pyrexia (24%), lymphopenia (22%) and abdominal pain (21%).
Drug Interactions
Avoid coadministration of VITRAKVI with strong CYP3A4 inhibitors (including grapefruit or grapefruit juice), strong CYP3A4 inducers (including St. John’s wort), or sensitive CYP3A4 substrates. If coadministration of strong CYP3A4 inhibitors or inducers cannot be avoided, modify the VITRAKVI dose as recommended. If coadministration of sensitive CYP3A4 substrates cannot be avoided, monitor patients for increased adverse reactions of these drugs. For coadministration with moderate CYP3A4 inhibitors, monitor for adverse reactions more frequently and reduce the dosage based on severity. For coadministration with moderate CYP3A4 inducers, modify dose as recommended.
Use in Specific Populations
Lactation: Advise women not to breastfeed during treatment with VITRAKVI and for 1 week after the last dose.
For important risk and use information about VITRAKVI, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of products to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
For Bayer products, you can report these directly to Bayer by clicking here.
Reference:
- VITRAKVI [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc.; March 2021. Return to content
© 2023 Bayer. All rights reserved. Bayer, the Bayer Cross, and VITRAKVI are registered trademarks of Bayer, and Access Services by Bayer is a trademark of Bayer. All other trademarks are the property of their respective owners.
This site is intended for US audiences only.
Site last modified: 11/2023


