Across NTRKa gene fusion–positive solid tumors
3 OUT OF 4 PATIENTS RESPONDED TO TREATMENT WITH VITRAKVI® (larotrectinib)1
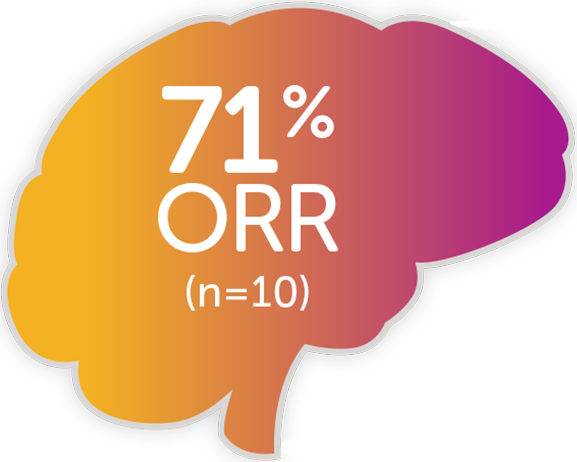
Robust response across solid tumors1

Rapid response2
Median time to best response was 1.84 months
(25/75 percentile: [1.77, 1.97]).
VITRAKVI is approved under accelerated approval based on overall response rate and duration of response. Continued approval for VITRAKVI may be contingent upon verification and description of clinical benefit in confirmatory trials.
Study design: Pooled efficacy analysis based on 3 open-label, single-arm clinical studies in adult and pediatric patients with unresectable or metastatic solid tumors with an NTRK gene fusion. All patients were required to have progressed following systemic therapy for their disease, if available, or would have required surgery with significant morbidity for locally advanced disease. Major efficacy outcome measures were ORR and DORc as determined by a BIRCc according to RECISTc v1.1.1
Consistent results in an expanded data set2,a,b